Re: Johnson&Johnson (JNJ): Seguimiento del valor
Novartis, Bayer... Y ahora esto. Cuando uno piensa en farmacia piensa en algo bueno. Pero parece ser que hay que ser cautos con estas empresas.
A ver como responde el valor
Resultados Johnson & Johnson 2Q 2019 y problemas legales
Lo más destacado de los resultados de Johnson & Johnson (2Q 2019)
- Los ingresos de JNJ han sido de 20,5B de dólares, lo que supone un descenso de un 1,3% respecto del año anterior.
- JNJ ha presentado en este segundo trimestre unos beneficios netos de 5,61B de dólares, lo que supone un incremento del 41,8% gracias a la sustancial rebaja en marketing y en gastos de administración.
- .Las ventas han aumentado en los segmentos más importantes de la empresa: prescripción médica (+1,7%), y en los medicamentos Imbruvica (+34.1%), Darzalex (+51.6%); y Stelara (+16.1%).
- Las ventas han caido en algunos segmentos como equipamiento médico para el diagnóstico (-6,5%).
Además de esto, el pasado viernes, el departamento de justicia de Estados Unidos anunció que investigaría a JNJ por presuntamente no informar al público sobre los posibles efectos cancerígenos de uno de sus talco para bebé.
¿Pensáis que está justificada la caída de casi un 7% con los problemas legales que han surgido? ¿Creéis que es una buena oportunidad para comprar más barata una empresa tan estable como es JNJ?
Re: Resultados Johnson & Johnson 2Q 2019 y problemas legales
Hace poco salió a los medios que JNJ iba a empezar a probar una nueva vacuna contra el VIH en humanos. Si les funciona bien el invento pueden pegar un buen pelotazo no?
https://elpais.com/sociedad/2019/07/12/actualidad/1562942536_177617.html
J&J sube el dividendo un 6%
· EPS of $2.17 increased 56.1%; adjusted EPS of $ 2.30 increased 9.5%*
· Dividend increase of 6.3% announced
· Long term fundamentals remain intact; 2020 guidance lowered to reflect COVID-19 impact and related investments
New Brunswick, N.J. (April 14, 2020) – Johnson & Johnson (NYSE: JNJ) today announced results for first-quarter 2020. The Company also announced earlier today that its Board of Directors declared a 6.3% increase in the quarterly dividend rate, from $0.95 per share to $1.01 per share. At the new rate, the indicated dividend on an annual basis is $4.04 per share compared to the previous rate of $3.80 per share.
“With Johnson & Johnson’s century-plus history of leading in times of great challenge, we are mobilizing our resources across the Company in the fight against the COVID-19 pandemic,” said Alex Gorsky, Chairman and Chief Executive Officer. “Johnson & Johnson is built for times like this, and we are leveraging our scientific expertise, operational scale and financial strength in the effort to advance the work on our lead COVID-19 vaccine candidate. We are committed to beginning production at risk imminently and bringing an affordable and accessible vaccine to the public on a not-for-profit basis for emergency pandemic use.”
Mr. Gorsky continued, “I am both proud and amazed at the level of dedication that I have witnessed from our more than 132,000 employees as we have focused on delivering on our commitments and responsibilities to the patients and consumers we serve. Our strong performance in the first quarter reflects the efforts of our teams around the world and the sustainability of our business model. Today, our Board of Directors approved an increase in our quarterly dividend for the 58th consecutive year, underscoring our commitment to delivering value for our shareholders and the confidence we have in our business now and in the future.”
OVERALL FINANCIAL RESULTS:

1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures included in accompanying schedules
2 Excludes the impact of translational currency
3 Excludes the net impact of acquisitions and divestitures and translational currency
4 Excludes intangible amortization expense and special items

1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures included in accompanying schedules
2 Excludes the impact of translational currency
3 Excludes the net impact of acquisitions and divestitures and translational currency
Note: values may have been rounded

1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures included in accompanying schedules
2 Excludes the impact of translational currency
3 Excludes the net impact of acquisitions and divestitures and translational currency
Note: values may have been rounded
SEGMENT COMMENTARY:
Consumer Health
Consumer Health worldwide operational sales, excluding the net impact of acquisitions and divestitures, grew 11.0%* driven primarily by over-the-counter products including TYLENOL and MOTRIN analgesics; upper respiratory products including ZYRTEC; digestive health products and ZARBEE’S NATURALS. Other contributors to growth were LISTERINE mouthwash in oral care products; NEUTROGENA and AVEENO in skin health/beauty products, as well as STAYFREE and o.b. in international women’s health. Consumer Health results across the majority of franchises were positively impacted by the increased demand related to the COVID-19 pandemic.
Pharmaceutical
Pharmaceutical worldwide operational sales, excluding the net impact of acquisitions and divestitures, grew 10.2%* driven by STELARA (ustekinumab), a biologic for the treatment of a number of immune-mediated inflammatory diseases, DARZALEX (daratumumab), for the treatment of multiple myeloma, IMBRUVICA (ibrutinib), an oral, once-daily therapy approved for use in treating certain B-cell malignancies, a type of blood or lymph node cancer, INVEGA SUSTENNA/XEPLION/INVEGA TRINZA/TREVICTA (paliperidone palmitate), long-acting, injectable atypical antipsychotics for the treatment of schizophrenia in adults, ERLEADA (apalutamide), a next-generation androgen receptor inhibitor for the treatment of patients with prostate cancer, OPSUMIT (macitentan), an oral endothelin receptor antagonist indicated for the treatment of pulmonary arterial hypertension to delay disease progression, TREMFYA (guselkumab), a biologic for the treatment of adults living with moderate to severe plaque psoriasis, and PREZISTA/PREZCOBIX/REZOLSTA/SYMTUZA for the treatment of human immunodeficiency virus type 1 (HIV-1) infection. This growth was partially offset by biosimilar and generic competition, with declines primarily in international VELCADE (bortezomib), a proteasome inhibitor for the treatment of multiple myeloma, REMICADE (infliximab), a biologic approved for the treatment of a number of immune-mediated inflammatory diseases, and PROCRIT (epoetin alfa), a treatment for chemotherapy-induced anemia and patients with chronic kidney disease.
Medical Devices
Medical Devices worldwide operational sales, excluding the net impact of acquisitions and divestitures, declined by 4.8%* driven by the estimated net negative impact of the COVID-19 pandemic and the associated deferral of medical procedures to our Surgery, Orthopaedics, Interventional Solutions and Vision businesses.
Resultados Q3
· EPS of $1.33 increased 101.5%; adjusted EPS of $2.20 increased 3.8%*
· Company increasing guidance for Full Year Reported Sales by $1.0 billion and Adjusted EPS by $0.15 driven by the strength of the recovery and strong underlying business fundamentals

1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures included in accompanying schedules
2 Excludes the impact of translational currency
3 Excludes the net impact of acquisitions and divestitures and translational currency
4 Excludes intangible amortization expense and special items

1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures included in accompanying schedules
2 Excludes the impact of translational currency
3 Excludes the net impact of acquisitions and divestitures and translational currency
Note: values may have been rounded
1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures included in accompanying schedules
2 Excludes the impact of translational currency
3 Excludes the net impact of acquisitions and divestitures and translational currency
Note: values may have been rounded
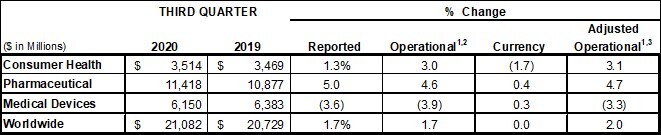
Consumer Health
Consumer Health worldwide operational sales, excluding the net impact of acquisitions and divestitures, increased by 3.1%*, inclusive of the net negative impact of COVID-19 primarily in international over-the-counter products. Sales growth was driven by U.S. growth in over-the-counter products including TYLENOL analgesics and digestive health products; LISTERINE mouthwash in oral care products; OGX in skin health/beauty products; and wound care products, primarily BAND-AID® Brand Adhesive Bandages.
Pharmaceutical worldwide operational sales, excluding the net impact of acquisitions and divestitures, grew 4.7%* driven by DARZALEX (daratumumab), for the treatment of multiple myeloma, STELARA (ustekinumab), a biologic for the treatment of a number of immune-mediated inflammatory diseases, IMBRUVICA (ibrutinib), an oral, once-daily therapy approved for use in treating certain B-cell malignancies, a type of blood or lymph node cancer, INVEGA SUSTENNA/XEPLION/INVEGA TRINZA/TREVICTA (paliperidone palmitate), long-acting, injectable atypical antipsychotics for the treatment of schizophrenia in adults, OPSUMIT (macitentan), an oral endothelin receptor antagonist indicated for the treatment of pulmonary arterial hypertension to delay disease progression, UPTRAVI (selexipag), an oral prostacyclin receptor agonist used to treat pulmonary arterial hypertension and reduce hospitalization, and ERLEADA (apalutamide), a next-generation androgen receptor inhibitor for the treatment of patients with prostate cancer. This growth was partially offset by the negative impact of COVID-19 as well as biosimilar and generic competition, with declines primarily in REMICADE (infliximab), a biologic approved for the treatment of a number of immune-mediated inflammatory diseases, and ZYTIGA (abiraterone acetate), an oral, once-daily medication for use in combination with prednisone for the treatment of metastatic castration-resistant prostate cancer.
Medical Devices worldwide operational sales, excluding the net impact of acquisitions and divestitures, declined by 3.3%*. The decline was primarily driven by the negative impact of the COVID-19 pandemic and the associated deferral of medical procedures to our Surgery, Orthopaedics, and Vision businesses. Results reflect market recovery versus the second quarter. The decline was partially offset by growth in the Interventional Solutions business led by electrophysiology products.
